Using Ionic Formula Puzzle Pieces to Model Net Ionic Equations
Do your students struggle visualizing net ionic equations? Do they have writing the ratio of cations to anions when they break strong electrolytes apart? How about writing Cl₂ when they really should be writing Cl⁻? I’ve seen all of these, and I’ve really focused this year on diagrams and models, hoping that those would help students make the jump to net ionic equations easier. And I think it might be working. I started net ionic equations with a station model that didn’t really have much information about net ionic equations at all, but instead focused on models of strong electrolytes, and the ratio of cations to anions. The next class period, we jumped into net ionic equations. And in addition to just writing the net ionic equations, we spent time modeling the strong electrolytes and the solid precipitates as well. One of those modeling activities had me digging out my ionic formula puzzle pieces. You know…those pieces we use when we’re teaching students how to form ionic formulas. In this post, I’m going to outline how I walked my students through an example using the puzzle pieces and chalk markers, before I had students try a couple of additional examples on their own.
Step 1: Writing the Reaction.
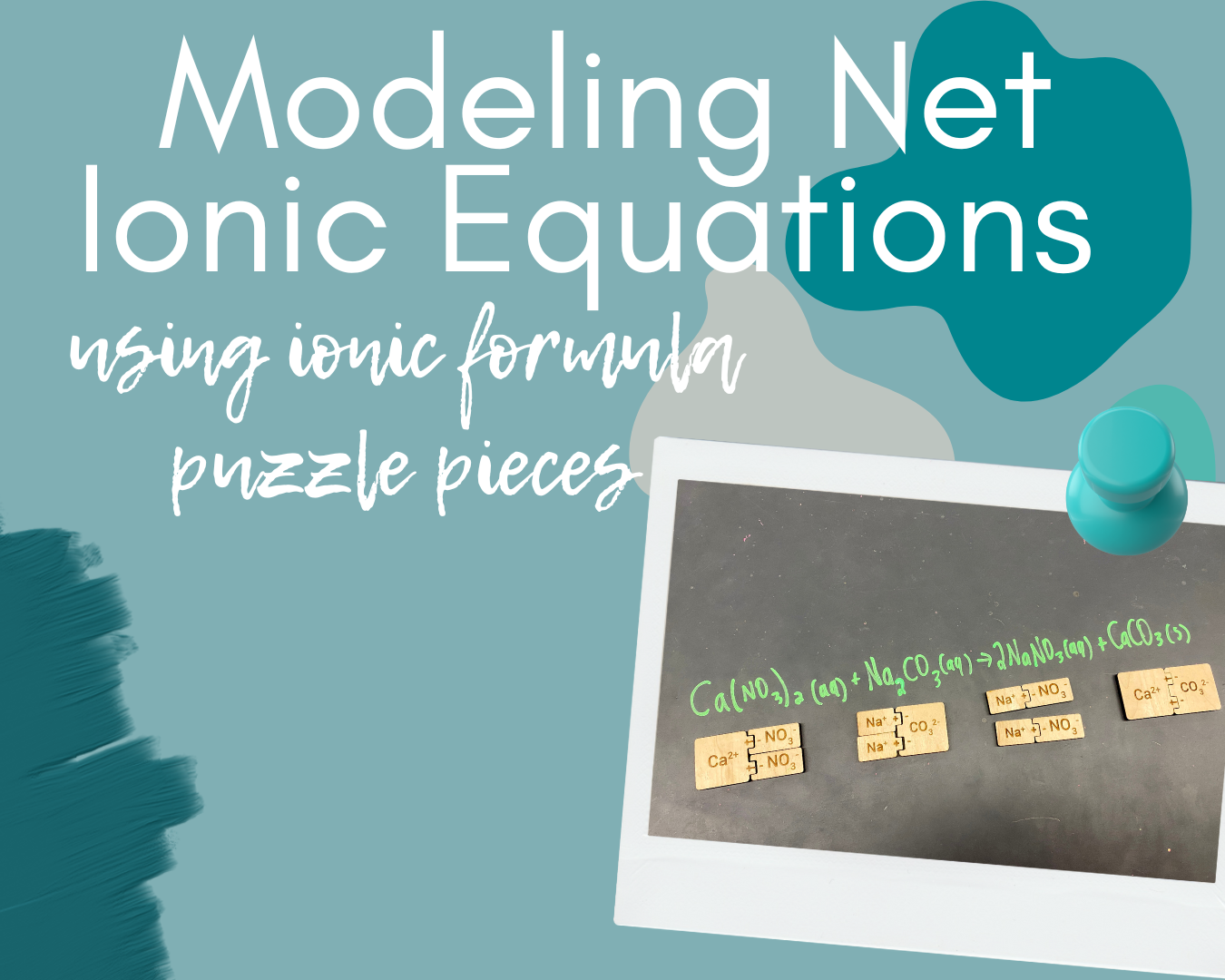
I began with having students pair up, and asked them to grab a chalk marker. I projected reactants on the screen, which I just typed into a Google Slides presentation. For our first reaction, this was “calcium nitrate + sodium carbonate.” I asked students to work with their partners to predict the products, write and balance the equation, and predict states of matter. As students worked in pairs, I roamed around the room to make sure those reactions were written properly.
Step 2: Modeling the Reaction.
Each pair procured a container with the ionic puzzle pieces. I asked students to model the reaction using the puzzle pieces. This went well, but here were two mistakes I noticed, although not frequently:
One group had one Na⁺ with the CO₃²⁻. I had to point out to them that their formula indicated they need two Na⁺, and also that their puzzle was not complete with only one of each.
A few groups needed to be reminded that 2 NaNO₃ compounds would be produced per the balanced chemical equation.
Step 3: Strong Electrolytes.
Once they have the pieces together and modeled, I ask the partner pairs to discuss which substances are strong electrolytes. I then instructed the students to break apart (physically) the puzzle pieces for those substances that are strong electrolytes. Because we had done some modeling previously, almost all groups kept the solid together and ionized the strong electrolytes. After physically separating the puzzle pieces, I asked the partner pairs to now write the complete ionic equation based on their model.
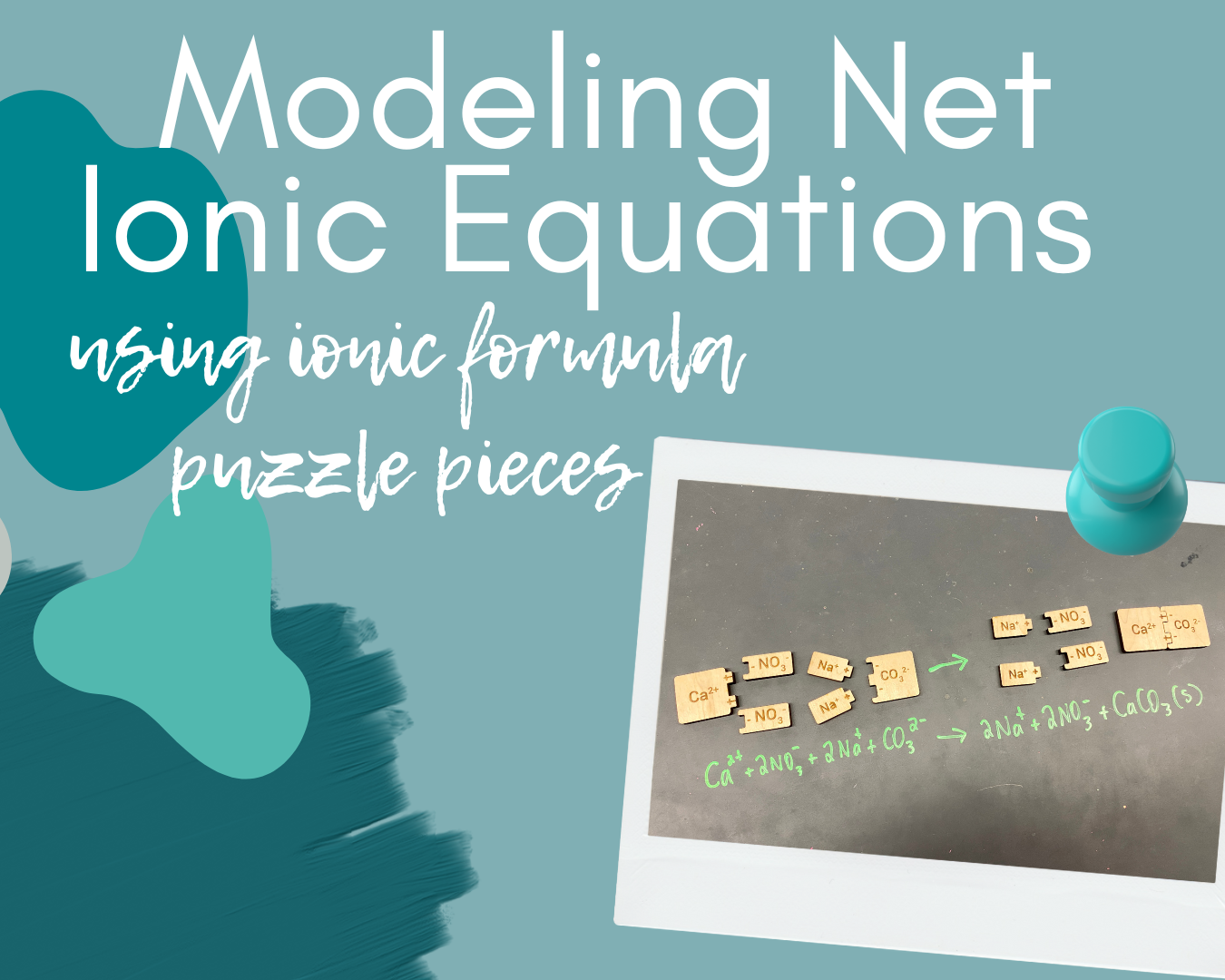
Step 4: Removing Spectators.
Next, I asked students to look at their model and the written equation and identify any spectators. Students were asked to both physically remove the spectators from their puzzle pieces and mark them out in their written net ionic equation.
Step 5: The Net Ionic Equation.
After the students physically remove the puzzle pieces that are spectators, what remains should only be those ions involved in the chemical reaction. The final step is asking thee students to write the net ionic equation. Throughout all of these steps, I circulated the room to monitor progress. After we had completed the first example, I had students complete these steps for two more examples. I simply projected the reaction I wanted them to model on the screen, and then changed it to the third reaction once most students had completed the second example.
From observations I made during this activity, I think this modeling was helping students transition to the net ionic equation. I witnessed far fewer students breaking apart the precipitate. I saw more students properly finding the cation to anion ratio when they broke apart ions in the ionic equations. What other activities could you use these iconic formula pieces with? Have you used them in a way that has been beneficial to your students? Let me know on Instagram. Thanks so much for reading!