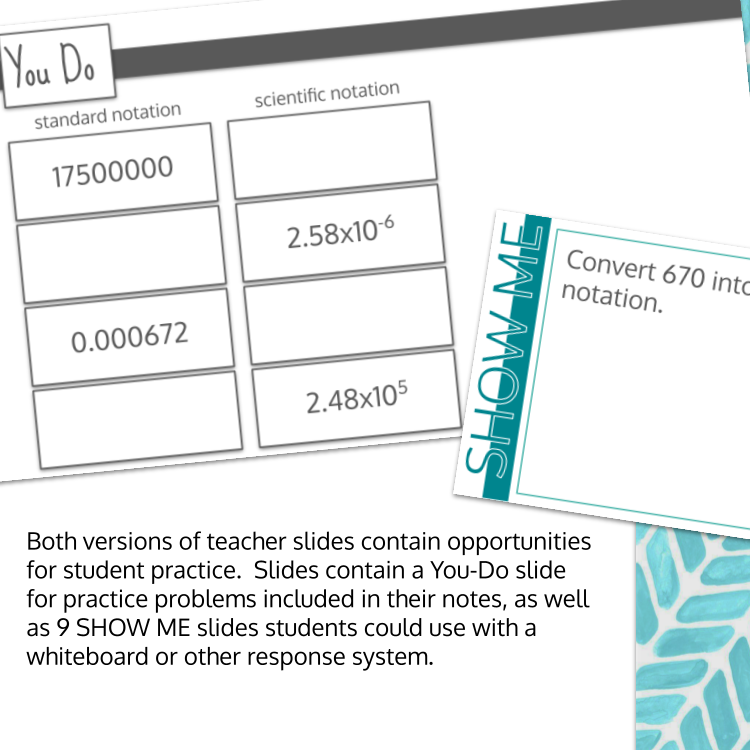
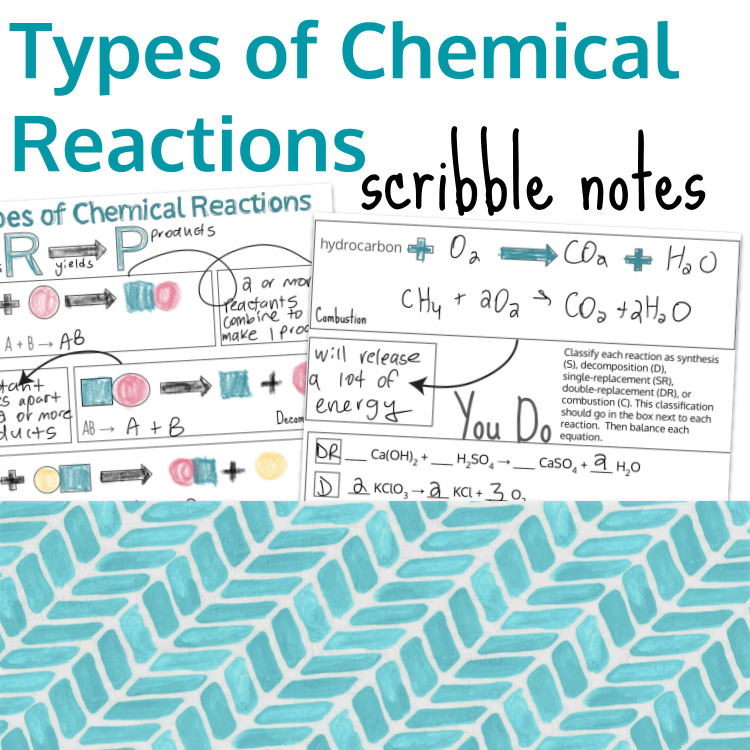
 Image 1 of 4
Image 1 of 4

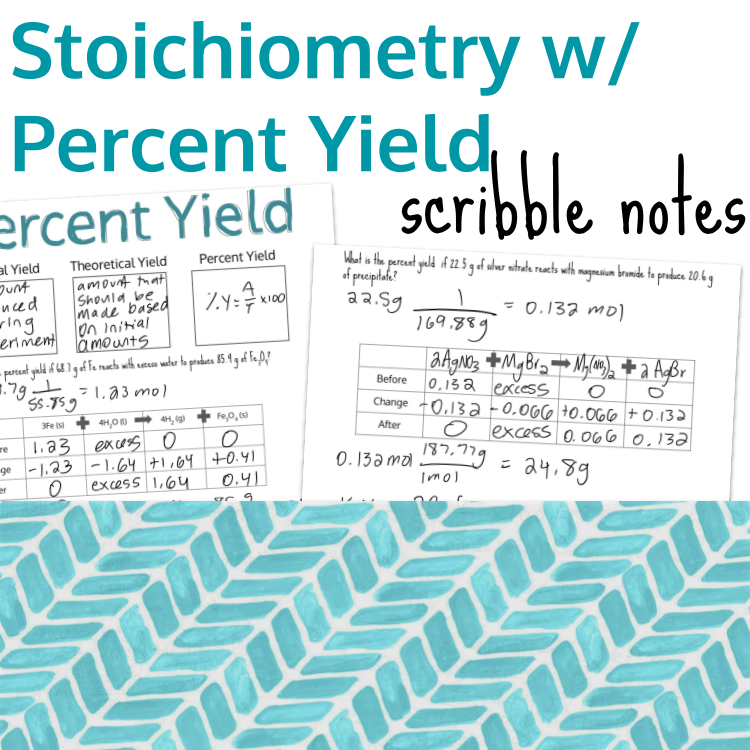
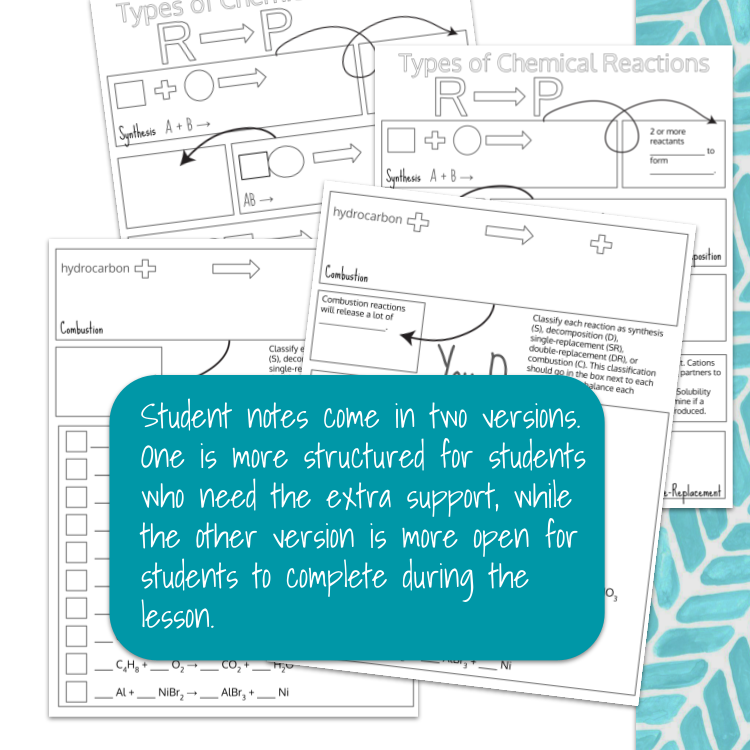
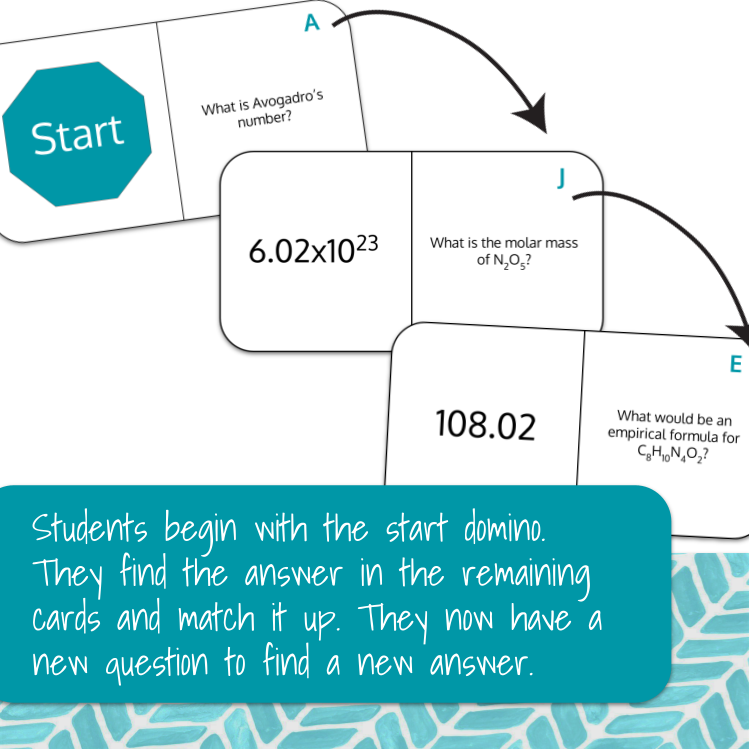 Image 2 of 4
Image 2 of 4

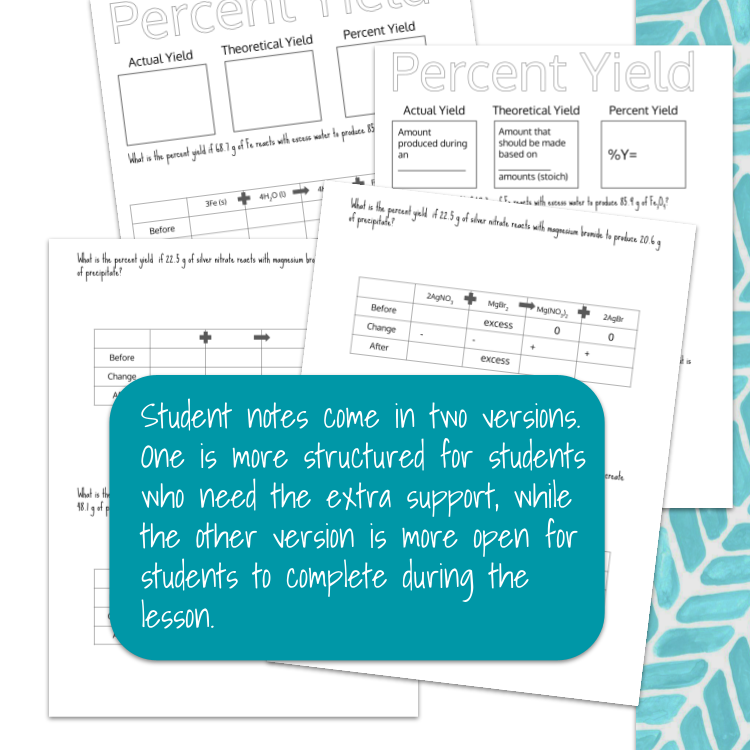
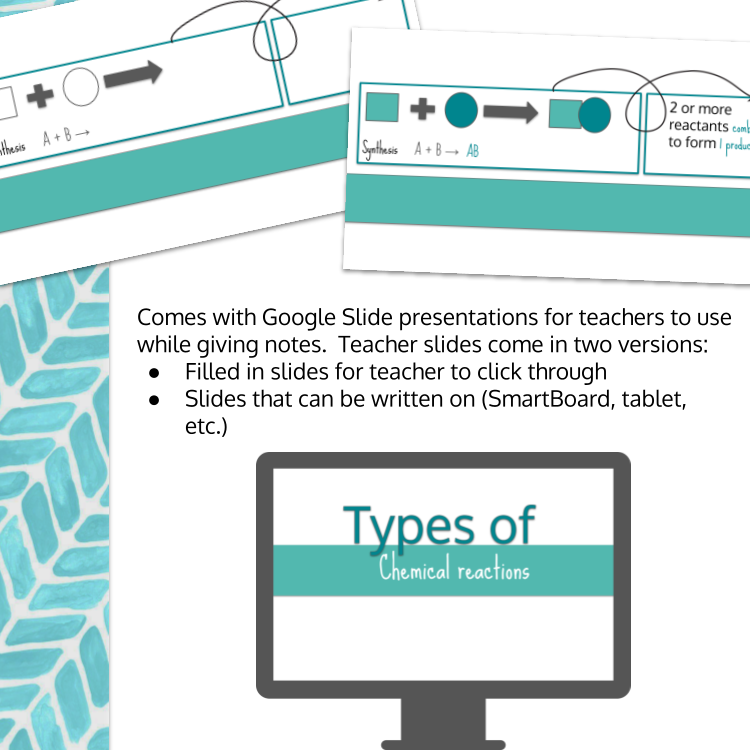
 Image 3 of 4
Image 3 of 4

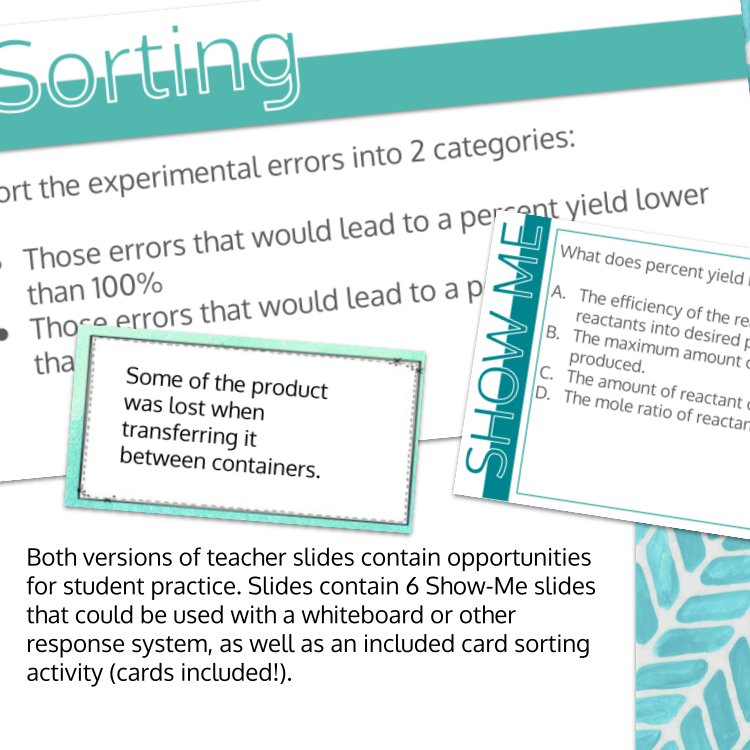
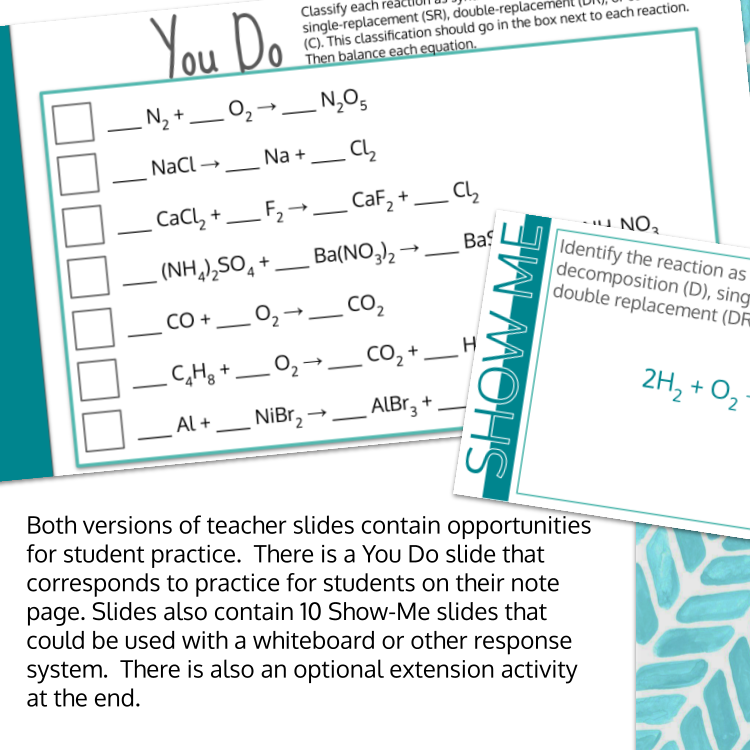
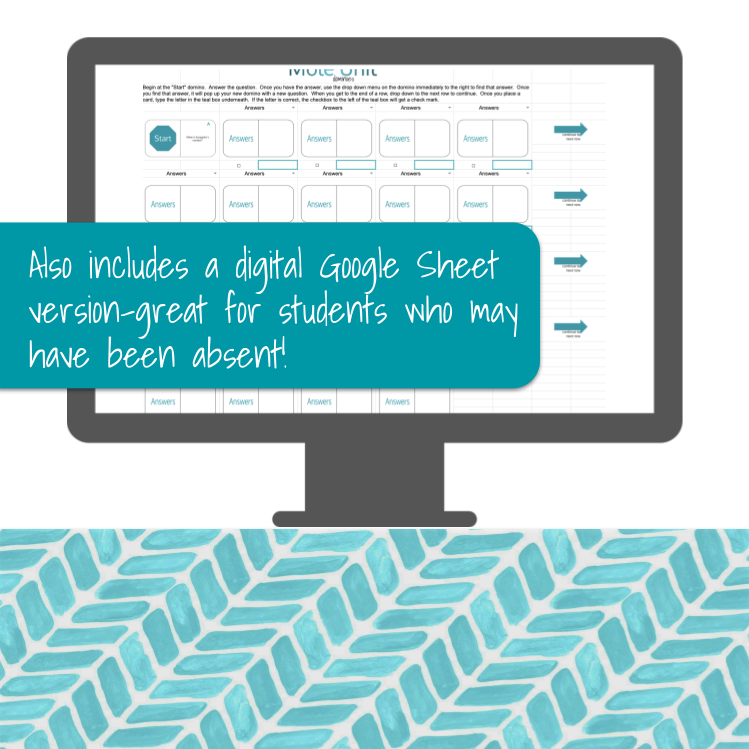 Image 4 of 4
Image 4 of 4





Chemistry Mole Empirical Formulas Dominoes Print Digital Self Check Activity
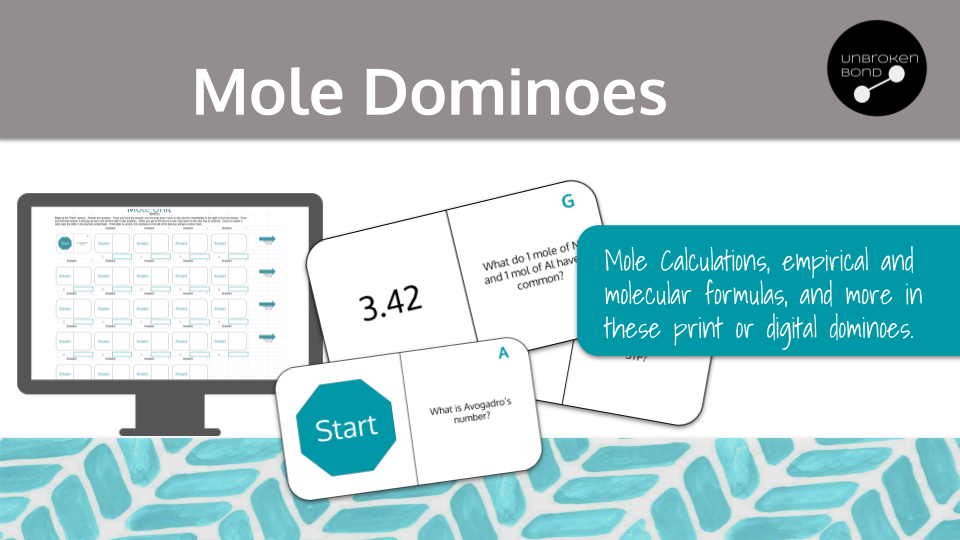
Dominoes is an engaging practice structure in which your chemistry students can practice content. This dominoes set contains 24 mole unit dominoes for students to practice a variety of calculations and concepts included in a mole unit. The following concepts and calculations are included:
1 and 2-step mole calculations involving moles, grams, liters at STP, and atoms
using percents to determine the empirical formula of a compound or molecule
determining percent composition
determining a molecular formula when given the empirical and a molar mass
definitions of a mole (Avogadro's number) and volume of a gas at STP
This resource can be used for review in class, as homework, etc.
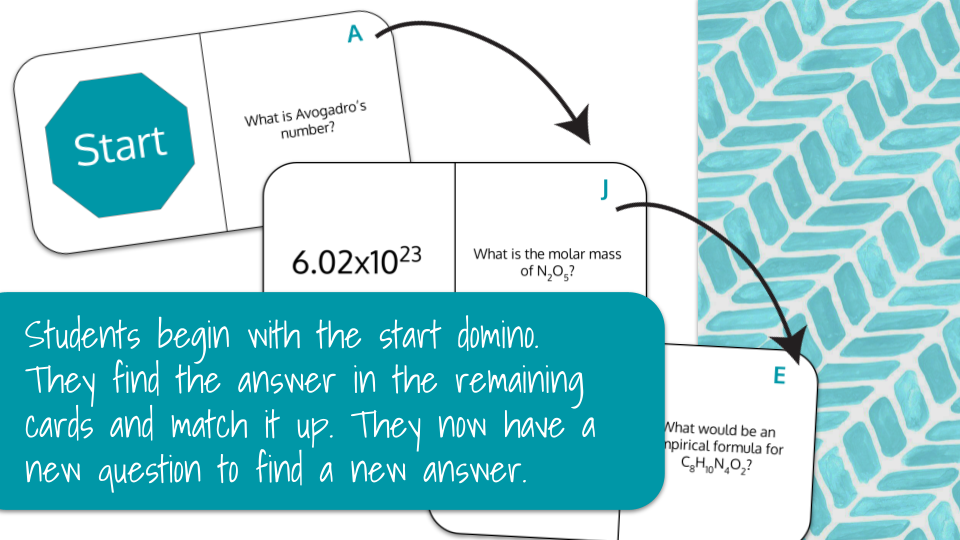
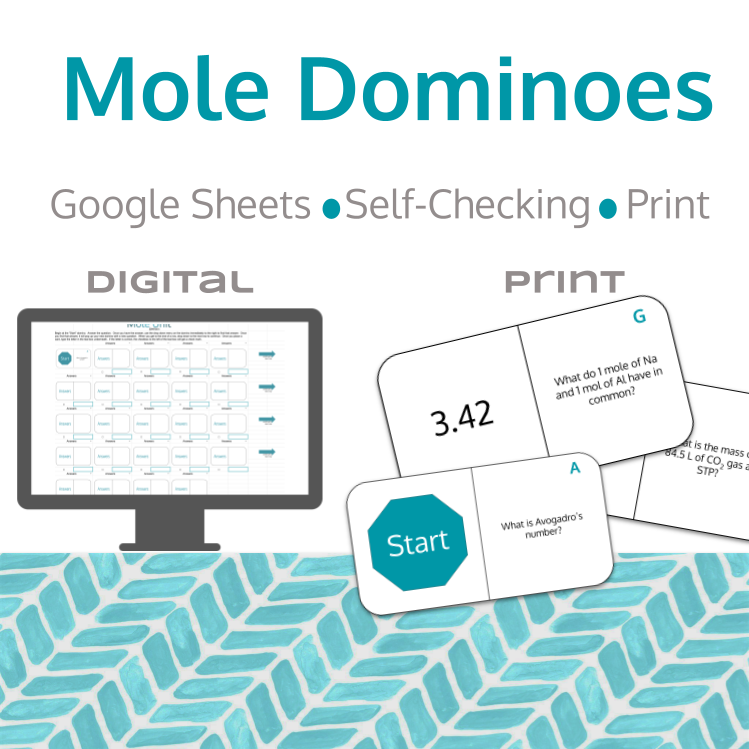
For the print version, students will begin with all sides flipped to the answer side. Students will begin at the "Start" card, where they will work the problem on the right side. Once they solve the problem, they will find the answer in the remaining cards. As in dominoes, the answer will align with the question. Once it is aligned, there is now a new question for the students to answer.
For the digital version, the process is very similar, except it is in a Google Sheet. Students will flip cards (to match the dominoes up), but there is no moving the cards around. The Google Sheet also has a way for students to check their answers as they proceed. Both you and your students must have a Google account and access to the internet to use this resource.
This resource includes both a print version, along with the student Google Sheet for the digital version.
Join my email list and receive four self-checking ideas, along with four FREE self-checking products and/or templates.
Let's connect on Instagram.
Dominoes is an engaging practice structure in which your chemistry students can practice content. This dominoes set contains 24 mole unit dominoes for students to practice a variety of calculations and concepts included in a mole unit. The following concepts and calculations are included:
1 and 2-step mole calculations involving moles, grams, liters at STP, and atoms
using percents to determine the empirical formula of a compound or molecule
determining percent composition
determining a molecular formula when given the empirical and a molar mass
definitions of a mole (Avogadro's number) and volume of a gas at STP
This resource can be used for review in class, as homework, etc.
For the print version, students will begin with all sides flipped to the answer side. Students will begin at the "Start" card, where they will work the problem on the right side. Once they solve the problem, they will find the answer in the remaining cards. As in dominoes, the answer will align with the question. Once it is aligned, there is now a new question for the students to answer.
For the digital version, the process is very similar, except it is in a Google Sheet. Students will flip cards (to match the dominoes up), but there is no moving the cards around. The Google Sheet also has a way for students to check their answers as they proceed. Both you and your students must have a Google account and access to the internet to use this resource.
This resource includes both a print version, along with the student Google Sheet for the digital version.
Join my email list and receive four self-checking ideas, along with four FREE self-checking products and/or templates.
Let's connect on Instagram.