Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



AP Chemistry Reaction Rates and Rae Law Mole Hill in Print and Digital
If you’re looking for a quick activity for students to practice reactions rates and rate laws, try out this kinetics mole hill for AP Chemistry. In this activity, students will practice:
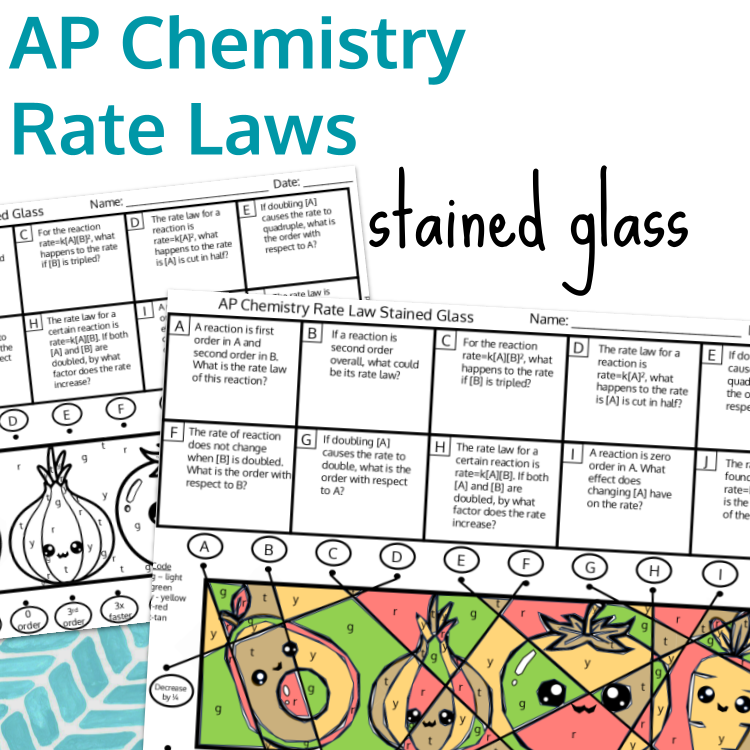
Identifying rate laws based on reaction orders
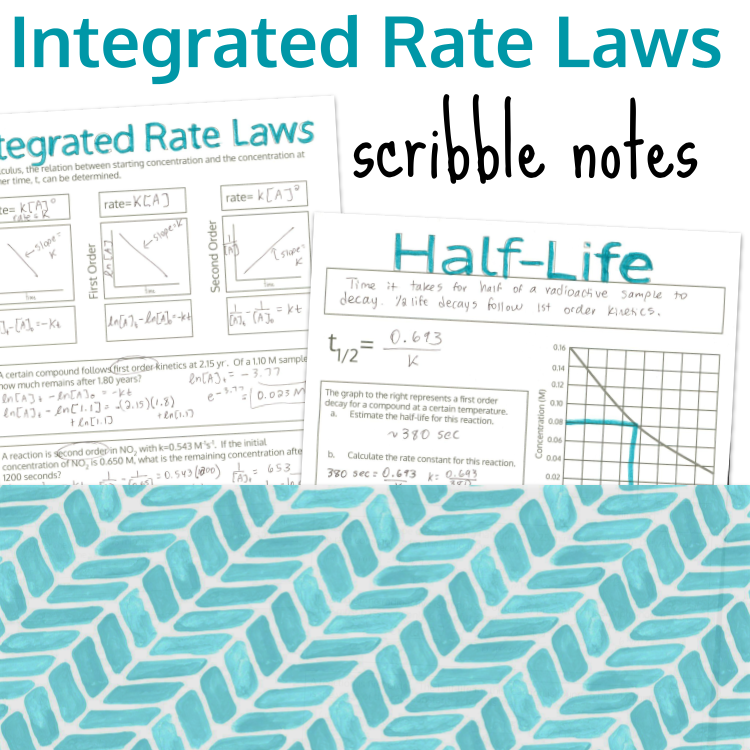
Calculating the rate of a reaction by calculating the change in concentration over the change in time
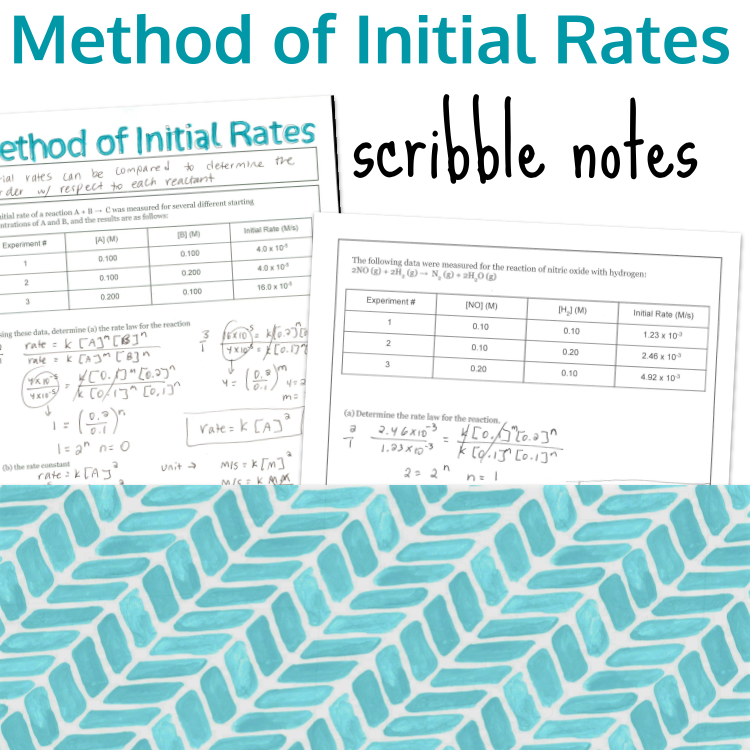
Calculating the rate of appearance or disappearance for a reaction using mole ratios
This resource can be used for distance learning, or as guided practice after in-class instruction, or for review.
For the print version, students will begin with all sides flipped to the answer side. Students will choose one card at random to flip to the question side, and then will answer that question. The answer to the first question will be in the answer bank somewhere, and when they find it, they flip that card over to the question side, placing it on top of the previous question. This forms a "mole hill," or question pile/question stack. The activity is very similar to dominos, except the students will stack their work. You will need to print out and cut the cards in enough sets for pairs of students to complete the activity. I also recommend laminating the cards for durability.
For the digital version, the process is very similar, except it is in a Google Sheet. Students will flip cards, but there is no stacking. Both you and your students must have a Google account and access to the internet to use this resource.
This resource includes both a print version, along with the student Google Sheet for the digital version.
Join my email list and receive four self-checking ideas, along with four FREE self-checking products and/or templates.
Let's connect on Instagram.
If you’re looking for a quick activity for students to practice reactions rates and rate laws, try out this kinetics mole hill for AP Chemistry. In this activity, students will practice:
Identifying rate laws based on reaction orders
Calculating the rate of a reaction by calculating the change in concentration over the change in time
Calculating the rate of appearance or disappearance for a reaction using mole ratios
This resource can be used for distance learning, or as guided practice after in-class instruction, or for review.
For the print version, students will begin with all sides flipped to the answer side. Students will choose one card at random to flip to the question side, and then will answer that question. The answer to the first question will be in the answer bank somewhere, and when they find it, they flip that card over to the question side, placing it on top of the previous question. This forms a "mole hill," or question pile/question stack. The activity is very similar to dominos, except the students will stack their work. You will need to print out and cut the cards in enough sets for pairs of students to complete the activity. I also recommend laminating the cards for durability.
For the digital version, the process is very similar, except it is in a Google Sheet. Students will flip cards, but there is no stacking. Both you and your students must have a Google account and access to the internet to use this resource.
This resource includes both a print version, along with the student Google Sheet for the digital version.
Join my email list and receive four self-checking ideas, along with four FREE self-checking products and/or templates.
Let's connect on Instagram.